Hello guys! Today I have before you another science experiment on the topic of electricity. The experiment finds out the conductivity ability of water.
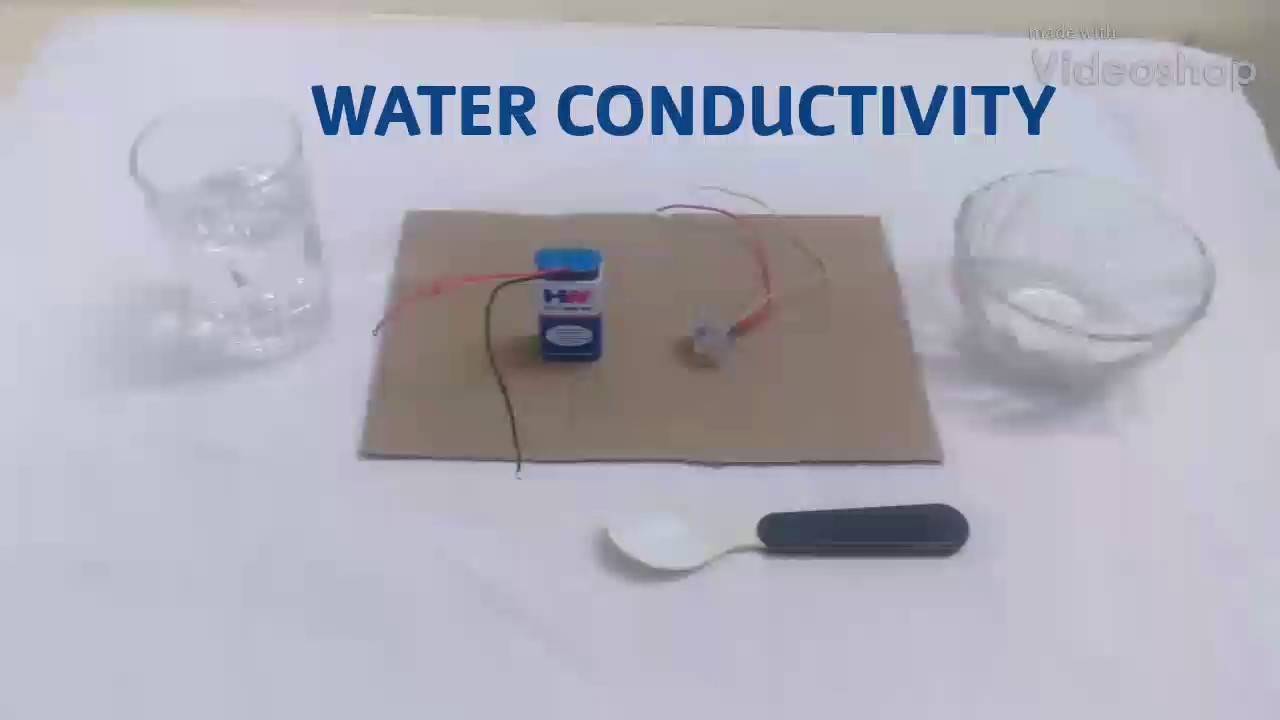
Materials required:
1. An electrical object (eg. LED bulb)
2. A Battery
3. A glass of pure water
4. A bowl of salt
5. A spoon
Procedure:
1. Take a battery and connect it to the electrical object.
2. Put the ends of the battery and object in a glass of pure water. You will observe that the object will not show electrical effect (in case of LED bulb, there is an effect, because LED bulb has less electrons).
3. Now remove the wires and add a bowl of salt to the water and stir it well until the salt dissolves in the water.
4. Now if you put the wires in the glass, the object will show much of electrical effect.
Explanation:
The bulb glowed only when salt was added to water, because salt (NaCl) contains Na+ and Cl- ions. When electricity passed through the salt solution, salt got dissociated into Na+ and Cl- ions. As a result, the Na+ ions start moving towards the negative terminal or cathode and the Cl- ions start moving towards the positive terminal or anode. When Na+ ions reach cathode, they gain electrons and when Cl- ions reach anode, they lose electrons. Hence, the electrons start moving inside the salt solution and starts passing through them and the bulb glows. On the contrary, pure water does not contain any electrons and hence the circuit was incomplete for the bulb to glow properly.
Thanks for watching the video. Please subscribe to my channel for more updates. Please do like and share the video.
Materials required:
1. An electrical object (eg. LED bulb)
2. A Battery
3. A glass of pure water
4. A bowl of salt
5. A spoon
Procedure:
1. Take a battery and connect it to the electrical object.
2. Put the ends of the battery and object in a glass of pure water. You will observe that the object will not show electrical effect (in case of LED bulb, there is an effect, because LED bulb has less electrons).
3. Now remove the wires and add a bowl of salt to the water and stir it well until the salt dissolves in the water.
4. Now if you put the wires in the glass, the object will show much of electrical effect.
Explanation:
The bulb glowed only when salt was added to water, because salt (NaCl) contains Na+ and Cl- ions. When electricity passed through the salt solution, salt got dissociated into Na+ and Cl- ions. As a result, the Na+ ions start moving towards the negative terminal or cathode and the Cl- ions start moving towards the positive terminal or anode. When Na+ ions reach cathode, they gain electrons and when Cl- ions reach anode, they lose electrons. Hence, the electrons start moving inside the salt solution and starts passing through them and the bulb glows. On the contrary, pure water does not contain any electrons and hence the circuit was incomplete for the bulb to glow properly.
Thanks for watching the video. Please subscribe to my channel for more updates. Please do like and share the video.
Comments
Post a Comment